Internal Energy
Internal Energy - The sum of the average kinetic and potential energies of a system’s constiuents.
Thermal Energy - The average kinetc energy of a system’s constiutents due to motion.
Increasing Internal Energy
The internal energy can be increased by doing work on the system / transferring energy to the system. One way of ‘doing work’ is to increase the temperature of the system.
The Absolute Temperature (Kelvin) Scale
There is a theoretical temperature called ‘Absolute Zero’ at which a substance would have zero internal energy (thus no kinetic energy; everything just stops). Due to laws to do with quantum mechanics and entropy, said temperature is impossible to accomplish and is a purely theoretical construct which is gained by extrapolating experimental data of kinetic energy against temperature.
You most likely are very familiar with the Celsius scale (). For example you should know that water boils at . On the Celsius scale, absolute zero is at approximately 1.
The Kelvin Scale (named after Lord Kelvin who proposed it) is a temperature scale positioned so that abolute zero is at .
Therefore to switch a temperature from Celsius to Kelvin, one must add 273.
Practice Questions:
Changes of State
During a change of state of a substance, there is a change in internal energy.
Before you continue, pause to think about the question below, then expand it to see the answer and explanation.
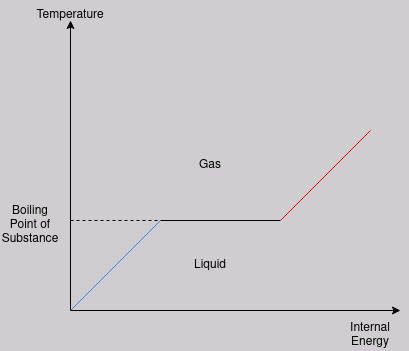
The above graph shows the relationship between temperature and internal energy as some generic substance, let us call it substance Q, is heated.
Temperature increases proportionally to internal energy until the boiling point, when there is an increase in internal energy but no increase in temperature. This increase in internal energy, as has been discussed, is due to an increase in potential energies.
Once the bonds have been broken, or at least ‘weakened’ enough for a change of state to occur, the substance returns to heating up as its internal energy is increased.
-
to be more precise. ↩︎