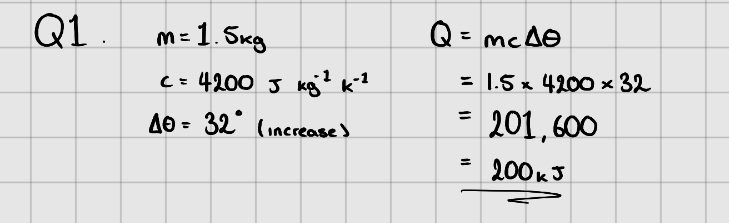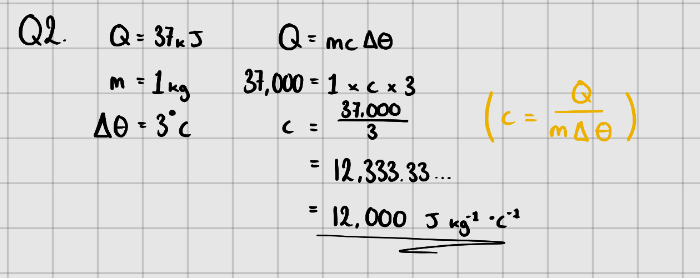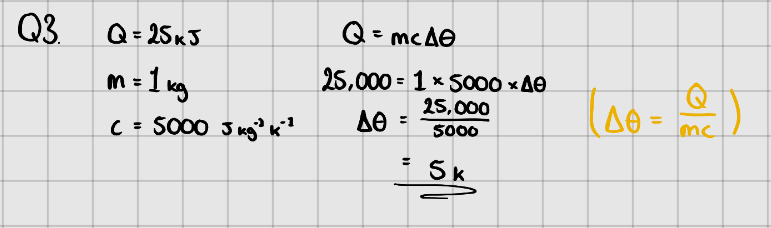Specific Heat Capacity
Specific Heat Capacity - The amount of energy needed to increase the temperature of 1kg of a (pure) substance by or .
Equation:
Where, - Change in energy ()
- Mass of substance (kg)
- Specific Heat Capacity ( or )
- Temperature change in or