Molecular and Molar Masses
Molecular Mass
The molecular mass is the total mass of a single molecule; it is the sum of the masses of all the atoms that make it up.
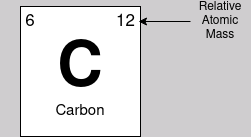
You should already have seen relative atomic masses being used in the periodic table. The way these are calculated is averaging the mass of the isotopes of an element, and placing it on a scale where Carbon-12 has a relative mass of 12.
The relative molecular mass of any one molecule is the sum of the relative atomic masses of all the atoms that make up the molecule.
Avogadro’s Constant
During the 19th century (1811), Amedeo Avogadro hypothesised that when a fixed volume of gas is kept at a fixed temperature, the gas would contain the same number of gas molecules whatever type of gas it was; any sample of gas kept at the same volume and pressure will contain an equal number of molecules.
Out of this hypothesis came the Avogadro Constant. It is denoted and is defined as the number of atoms in exactly of .
The Value of Avogadro’s Constant =
Molar Mass
1 mole of a substance is defined as a sample of that substance containing only atoms or molecules of that substance.
The molar mass is the usual mass of 1 mole of that substance, in grams. This mass is equal to either its relative atomic mass, or its relative molecular mass.
The number of moles in a mass of gas, denoted with units , when multiplied by Avogadro’s Constant gives the number of molecules in the mass of gas: