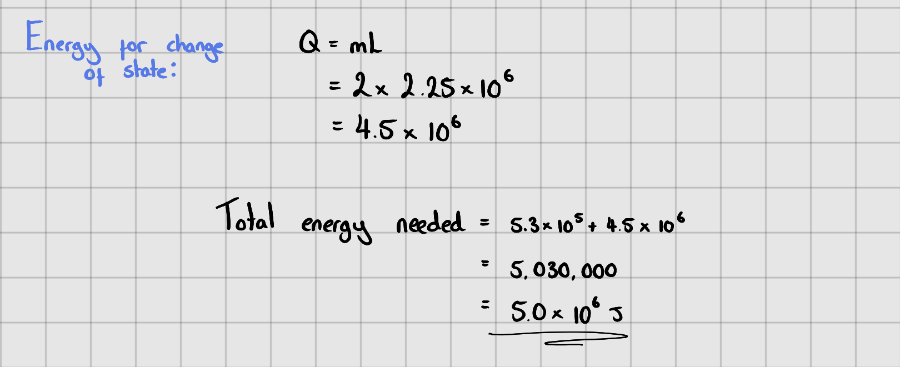Latent Heat Capacity
Equation
Where,
- Change in energy ()
- Mass of substance ()
- Specific latent heat ()
Explanation
The specific latent heat of a substance is the amount of energy required to change the state of 1kg of the material.
Latent heat capacities can be split further into two distinct types of latent heat capacity:
- Specific Latent Heat of Fusion - The work that must be done to convert 1 kg of a solid to a liquid
- Specific Latent Heat of Vaporisation - The work that must be done to convert 1kg of a liquid to a gas
It is very important to understand that the latent heat capacity does not consider the potential change in temperature needed so the substance can actually change state. It only accounts for the energy needed to change the state. To work out the energy needed to be supplied/removed for the increase/decrease of temperature needed, one must use specific heat capacity.
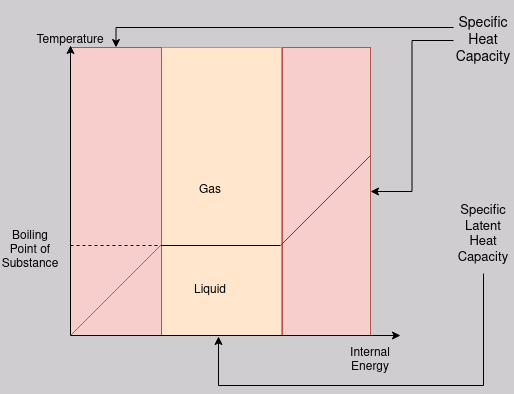
Below is the same graph as was in the Internal Energy notes, however this time it shows where the two types of heat capacities are ‘applicable’.
Since this graph shows the change of state between liquid and gas, you would have to use the latent heat of vaporisation.
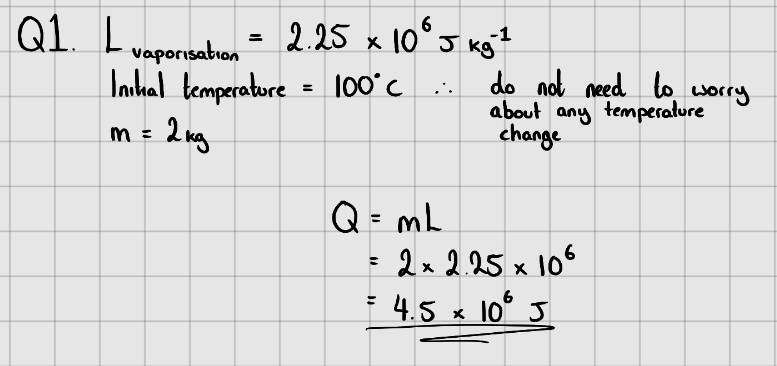
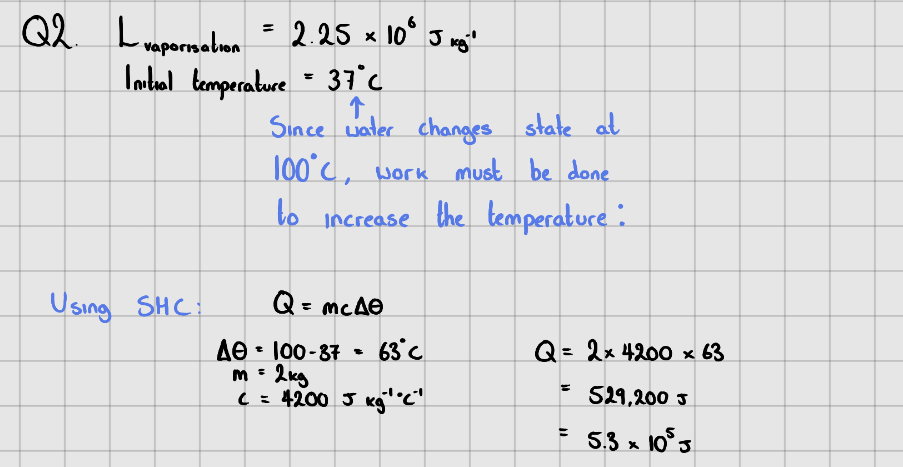
Starter Questions
Latent Heat of Vaporisation of Water =