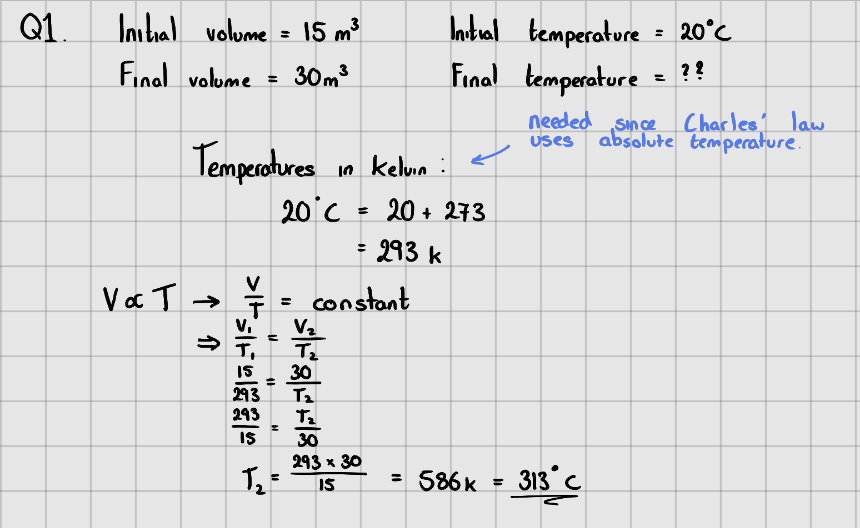
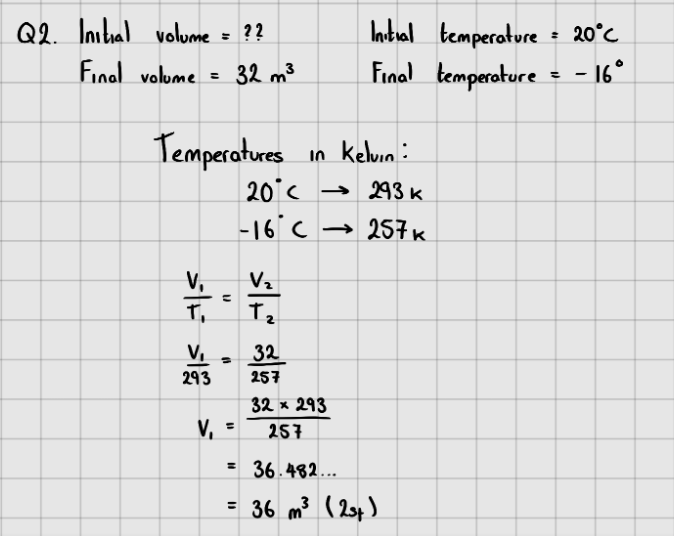
Gas Law II - Charles' Law
At a constant pressure, experiments show that the absolute temperature and volume of a gas are directly proportional.
$$\LARGE{V \propto T}$$
Where,
$V$ - volume of the gas
$T$ - temperature of the gas
Using this,
$$\LARGE{\frac{V}{T} = constant}$$