Rutherford’s Scattering Experiment
The Experiment:
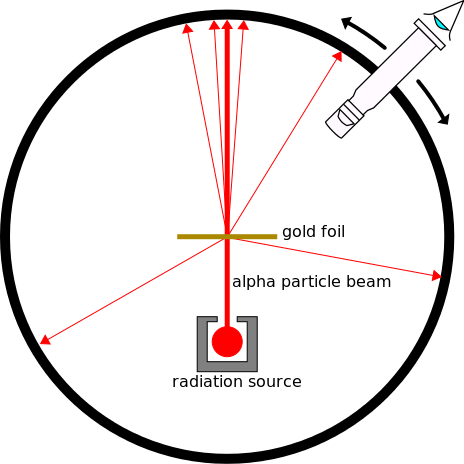
Image from: Kurzon - CC BY-SA 3.0 1
Alpha particles (particles with 2 protons and 2 neutrons, hence positively charged) are fired at very thin gold leaf. The angle of deflection is measured using a fluorescent screen encapsulating the radium source which lights up when impacted by an alpha particle. A microscope is used to view the paths of alpha particles.
Conclusions:
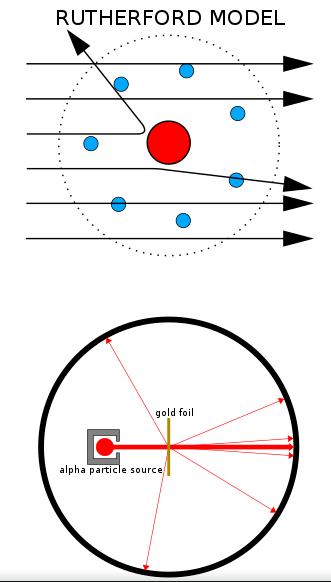
- The atom must be mostly empty space - This is because the majority of the alpha particles pass through the gold leaf unaffected.
- The nucleus must contain the majority of the mass of the atom - This is because for an alpha particles to be deflected in the way that was observed they must be impacting something more massive than themselves.
- The nucleus must have a large positive charge - This is because the positively charged alpha particles, when deflected, often are deflected by a very large angle.
- The nucleus must be very small - This is because very few of the alpha particles are deflected more than $90^{\circ}$.
Image cropped from: Kurzon - CC BY-SA 3.0 2