Key Points: Development of the Atomic Model
5th Century BC - Democritus
- He proposes that all matter is made up from very small, identical ‘lumps’ called ‘atomos’ - the Greek word for ‘indivisible’.
1804 - John Dalton
- Brings Democtritus’ idea back to scientific thinking of the time - he also believes that all matter is made up from small, indivisible constituents (he believed spheres).
- Proposes that each element has its own type of atom.
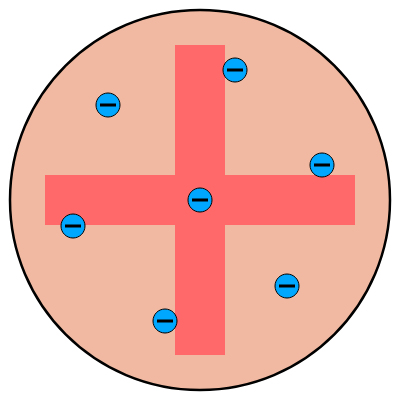
1904 - J.J. Thompson
- Thompson discovers electrons can be removed from atoms.
- This leads him to posit that an atom is made from a sphere of positive charge with electrons dotted in a distribution that gives the atom an overall neutral charge.
- The idea of electrons dotted about the sphere gave it the colloquial name ‘The Plum Pudding Model’.
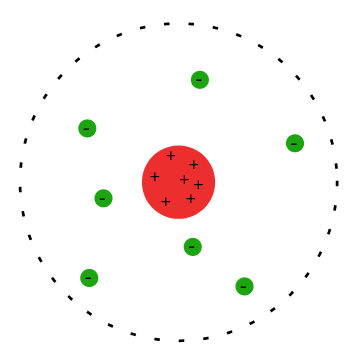
1909 - Rutherford (Geiger and Marsden)
- Alpha particles are fired at thin gold leaf. Any deflection of the alpha particles is measured.
- The majority of alpha particles pass straight through the gold leaf - this suggests the atom must be mostly empty space.
- Roughly 1 in 8000 alpha particles are deflected more than - this suggests the atom must have a small, dense and positively charged core; this became known as the atom’s nucleus.
- The experiment is known as the Rutherford Scattering Experiment.
1919 - Rutherford and Kay
- Rutherford and William Kay discover the neutron by accident whilst trying to determine the charges of high mass nuclei.
- The charges of very high mass nuclei were lower than expected, suggesting another particle must be present to give the higher masses.
- Rutherford proposes a ‘proton-electron doublet’; this idea would later come to fruition as what we now know as the neutron - proven through experiment by James Chadwick in 1932.
The Quantum Revolution
If you would like to better understand modern ideas of the atomic structure, and you have not already, feel free to read the previous page which goes beyond the specification of the A-Level course to provide a narrative look upon the topic.