The History of the Atom: Democritus to Schrödinger
The following is a narrative, a piece of writing in which the history of the atom is mapped out in a longer form. If you would prefer to see only the key points, please see the next page. If, however, you would enjoy reading an account of scientific history I hope you find pleasure in what follows.
It was during the time of the Ancient Greeks (5th Century BC) when Democritus, whose work has been lost to time and now is known of only though quotes in later works, proposed that all matter was made up of tiny, indivisible constituents. He called these ‘atomos’ - the word for ‘indivisible’ in the Greek language.
It was not until later, in 1804, that chemist John Dalton revived Democritus’ idea. He had been working on observing chemical reactions and the conservation of mass and this led him to propose his atomic theory. Almost identical to Democritus’, it theorised that atoms where tiny indivisible spheres. Dalton also made the presumption, which we now know to be correct, that each element was made up of a different type of atom. Bringing the theory back to scientific attention, it paved the way for the more modern atomic models that would follow.
Fast forward around 100 years, and the atomic model is on the verge of a massive breakthrough - one that would revolutionise Physics for ever.
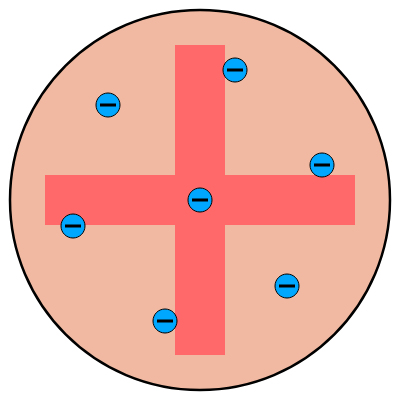
After discovering that electrons could be removed from an atom, J.J. Thomson set about designing a new atomic model. This was clearly needed since the discovery of electrons leaving these atoms went against the biggest assumption of Democritus’ and Dalton’s model: the atom was indivisible. Furthermore these electrons showed signs of an inherent negative charge whilst their ‘parent’ atoms did not. How could the atom be of neutral charge and yet be made up of negatively charged particles? Thomson suggested that an atom was like a plum pudding (indeed the model has been colloquially named ‘The Plum Pudding Model’) and consisted of a spherical positive charge with the negatively charged electrons dotted here and there so that the atom displayed a net neutral charge. This became the ‘Thomson Model’ and can be seen illustrated below:
It would only be another 5 years before Ernest Rutherford’s students, Hans Geiger and Ernest Marsden, would falsify this model.
This was done by shooting alpha particles (a particle consisting of two protons and two neutrons, hence bearing a positive charge) at very thin gold leaf. The alpha particles were emitted from radium sources and were aimed at gold leaf with thickness of approximately . If Thomson’s model was correct only very small deflections of the alpha particles would be expected. This is not what was observed and instead most alpha particles passed directly though the gold foil. Geiger and Marsden realised, however, that in some cases the alpha particles were deflected at angles greater than , some even rebounding directly in the direction of the source.
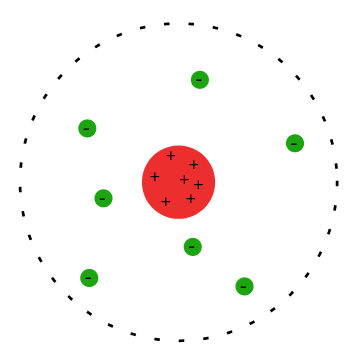
This behaviour of deflections suggested that the atoms in the gold leaf, and by induction all atoms, had a small and dense positively charged volume - this became known as the nucleus of the atom. It had to be positively charged to deflect the alpha particles and it had to be small since the majority of the particles (approximately 1 in 8000 were deflected past ) passed through the gold leaf uninterrupted. Electrons must be present still, Thomson’s observations of these were not wrong even if his ideas on the atom ultimately were, but how these electrons were positioned and organised was not full understood after the atomic nucleus was proven. The new models that resulted from what is now, somewhat unfairly, called ‘Rutherford’s Scattering Experiment’ looked something like this:
The next big name in the story is Niels Bohr. The father of Quantum Physics.
Bohr’s work on black-body radiation showed that energy was fundamentally quantized; it came in discrete packets. Spectrum of the light emitted when atoms were excited (additionally energy was transferred to them) was seen to be discrete. It was a logical conclusion to Bohr that the energy of electrons in an atom must, therefore, be of a quantum nature. He hypothesised that electrons could only ‘sit’ in certain energy levels / could only exist in certain energy states. These states corresponded to orbits around the nucleus. There was a fixed, immutable energy difference between these levels. An electron could absorb a photon carrying the energy of the gap, and jump to a higher energy level. It could also fall down, emitting a photon with the exact amount of energy lost by falling to a state with less energy; this explained why excited atoms only emitted certain wavelengths - there were only a limited number of energy levels an atom could jump between. Bohr’s atom looked as follows:
This model of the atom helped bring about the quantum revolution and the next major chapter of Physics.
Known by many a layman for his infamous cat in a box (which finds itself both alive and dead at the same time thanks to some formulations of quantum mechanics), Erwin Schrödinger was one of the most important contributors to the quantum ‘cookbook’, in which he gives his name to one of, if not the most important equations, the Schrödinger Equation. 1
It is not surprising then that he had his own idea on how the atom should be thought of.
Schrödinger removed the idea of orbiting electrons, indeed he removed the idea of particles being around the nucleus in general! Instead he said that electrons behave as waves around the nucleus and cannot be described in a way where one can say they know the exact position of the electron. The position of an electron in this model can only be said to be ‘more likely’ to be in a certain position than another. (If you are interested in seeing an attempt at a visual representation of this idea, may I suggest the youtube video by minutephysics: https://www.youtube.com/watch?v=W2Xb2GFK2yc. 2)
It is at this point that we have reached the end of our journey. While nuclear physicists still try to refine their understanding of the intricate subatomic structures of the atom, the ‘large-scale’ model of the entire atom has remained intact since Schrödinger, and is unlikely to change anytime soon. The story has shown us the volatility of science; how ground breaking discoveries push us ever closer to a true image of the atom, and yet how far we still are from truly understanding it.
-
1. The laws of quantum mechanics are often referred to as recipes in the ‘quantum cookbook’ since physicists use the laws without a full understanding of why they work - much like a novice baker following the recipe for some cupcakes. For a much wider description of this idea, I suggest ‘In Search Of Schrödinger’s Cat’ by John Gribbin. 2. You do not need to worry about understanding nor remembering this equation; it is degree level! ↩︎
-
Don’t worry if you find the video somewhat confusing, you won’t be question on this during your A-Level course. ↩︎