4.2.2 - Haloalkanes
Properties of Haloalkanes
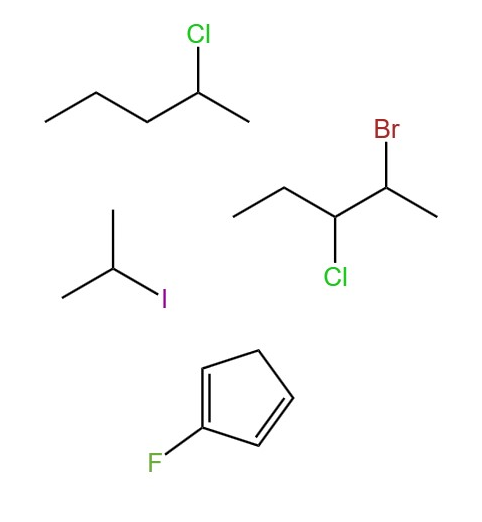
A haloalkane is another relatively simple saturated ‘non-hydrocarbon’. Similar to alcohols, haloalkanes have similar structures to regular alkanes, and differ in that one of more hydrogen atoms are replaced by a halogen. For example:
The properties and classification of haloalkanes are very similar to alcohols, and the same theory applies. The only difference between alcohols and haloalkanes in terms of bonding is that since there are no partially positive hydrogens in the functional group(s), hydrogen-bonding does not occur. This places the melting and boiling points of haloalkanes between those of alkanes and alcohols.
Substitution Reactions of Haloalkanes
Just as alcohols can be converted to haloalkanes through a substitution reaction, the reverse can happen in a similar way.
However, since the halogen group in a haloalkanes is less stable than the hydroxyl group in the alcohol, a halogen makes a good exiting/leaving group, and so no catalyst is required for the reaction to happen. The reagent is therefore just aqueous NaOH.
A second way to hydrolyse haloalkanes is to used water, ethanol and silver nitrate. This is an interest reagent mixture, but is used to compare experimentally the rates of hydrolysis of different carbon-to-halogen bonds. As explained in 3.1.4 - Qualitative Analysis, silver nitrate is used as the test for halide ions, and as the substitution reaction progresses, halide ions will become free in the mixture. The positive tests for the different ions will then appear.
However, the speed at which the positive test will appear depends or how quickly the carbon-halogen bonds in the molecules break. And the speed at which the carbon-halogen bonds break depends on the bond enthalpy.
Using deduction, the above simplifies to:
Which also means that the faster the precipitate forms in the reaction, the weaker the carbon-halogen bond.
The four carbon-halogen bond enthalpies in descending order are:
and is because of the atomic radius of the halogen atoms, and the relative attractions between the bonding pair and the nuclei. Fluorine atoms are the smallest halogen atoms, and so the distance between the fluorine nucleus and the bonding pair is least. This means that the electrostatic force between the shared pair of electrons and the nucleus (definition of a covalent bond) is strongest. The opposite is true for CI bonds, because iodine atoms are the largest.
The above paragraph is just the theory behind it. The extent of knowledge required for an exam may be: ‘the CF bond enthalpy is higher than the CCl, and so hydrolysis of a fluoroalkane requires more energy input - a higher activation energy - than of a chloroalkane. As a result, the rate of hydrolysis is slower for a fluoroalkane that a chloroalkane.’
NUCLEOPHILIC SUBSTITUTION:
The reaction mechanism for this reaction is required for this course. For it, an important definition is needed. An electron pair donor is called a nucleophile. This is the opposite of an electrophile.
In a mechanism, a nucleophile is drawn as a the chemical formula with a lone pair (which looks like a colon). Examples of a nucleophile include (note that the negative charges are shown):
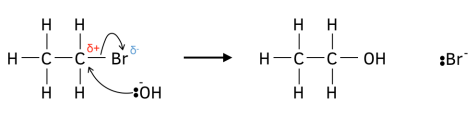
In the mechanism for nucleophilic substitution, a nucleophile such as a hydroxide ion attacks the partially positive carbon atom caused by the polar carbon-halogen bond. This forms a dative covalent bond. Simultaneously, (in the same step) since carbon cannot form 5 bonds, another bond must be broken. The bond that is broken is the most chemically active, i.e. the carbon-halogen bond.
Of course, in the drawing of the mechanism, lone pairs, curly arrows and partial charges must be shown for it to make chemical sense:
Environmental Concerns from Organohalogen Use
Halogens are relatively stable when they are covalently bonded to another atom. This is because they have a full electron energy level. However, their normal monoatomic states have one unpaired electron. You may recall that this is called a radical.
Radicals are highly reactive, and can be created by the action of UV radiation on halogen diatomic compounds and on halogenoalkanes in the upper atmosphere. This is an issue, because the protective blanket in the atmosphere that is the ozone layer is made up of ozone (O) which reacts with radicals to form oxygen gas, which means the ozone layer depletes.
The worst part about this is that the radical acts not as a reactant, but a catalyst, in that it is regenerated at the end of the reaction, so it can go on to cause more damage.
Below is a series of steps by which ozone is broken down by dichlorodifluoromethane (CFCl), a type of haloalkane called CFCs (chlorofluorocarbon) which were commonly used in aerosols, paints and healthcare products.