4.2.1 - Alcohols
Properties of Alcohols
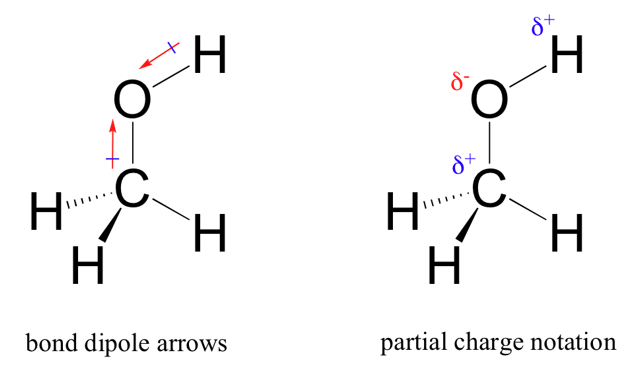
Alcohols are the simplest non-hydrocarbon organic molecule. They are compounds where a hydrogen has been replaced by a hydroxyl group, OH. This functional group is the first of many that contains a polar bond. In fact, there are two polar bonds.
These are the CO bond and the OH bond, where the oxygen atom is the most electronegative in both cases:
The introduction of polarity in organic molecules increases the number of intermolecular forces that occur between them. Having just any polar bond introduces permanent dipole-permanent dipole and permanent dipole-induced dipole interactions, but in addition to these, hydroxyl groups can make hydrogen bonds with each other.
As a result, alcohols have higher boiling and melting points than alkanes and alkene. They are also less volatile, meaning they are less likely to spontaneously evaporate.
CLASSIFICATION:
As with carbocations, alcohols are classified under three groups depending on the number of carbon atoms that a COH carbon binds to.
- Primary alcohols - an alcohol such that the hydroxyl group is bonded to a carbon that bonds with one other carbon.
- Secondary alcohols - an alcohol such that the hydroxyl group is bonded to a carbon that bonds with two other carbons.
- Tertiary alcohols - an alcohol such that the hydroxyl group is bonded to a carbon that bonds with three other carbons.
The class of alcohol determines which compounds can react with it, and the number of unique products.
Reactions of Alcohols
As with most organic compounds, alcohols are able to react with oxygen in a combustion react. The equations for complete and incomplete combustion of ethanol are shown below:
OXIDATION:
As well as combustion, alcohols can be oxidised to form a variety of products depending on the conditions applied during the oxidation, and the class of alcohol.
The oxidation of an alcohol requires an oxidising agent, as well as an acidic environment. The oxidising agent commonly used is potassium dichromate, with sulfuric acid as the source of hydrogen ions: H/CrO. In words, the reagent is known as acidified potassium dichromate.
In module 5, you will see that chromium undergoes a reduction during the reaction, and with that comes a colour change from orange to green. This fact can be used when trying to distinguish between tertiary and primary or secondary alcohols.
The reaction mixture is then distilled using a Liebig Condenser. If the acidified potassium dichromate is allowed to catalyse the reaction continuously, two reactions may happen. In this case, the condenser is upended so that any condensate drips back into the reaction vessel. This is called distilling under reflux. Otherwise, the products can be distilled immediately to prevent further oxidation.
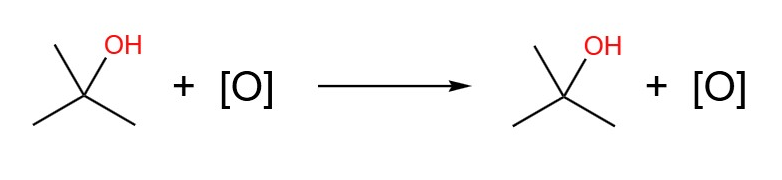
The table below shows the oxidation of the three classes of alcohol. Note that tertiary alcohols do not get oxidised. This is because the carbon that is bonded to the hydroxyl group is not also bonded to any hydrogens. Oxidation involves the removal of hydrogen, and so no hydrogens means no oxidation.
| Alcohol Class | Product under Immediate Distillation | Product under reflux |
|---|---|---|
| Primary | Aldehyde | Carboxylic Acid |
| Secondary | Ketone | Ketone (no further oxidation) |
| Tertiary | Tertiary alcohol (no change) | Tertiary alcohol (no change) |
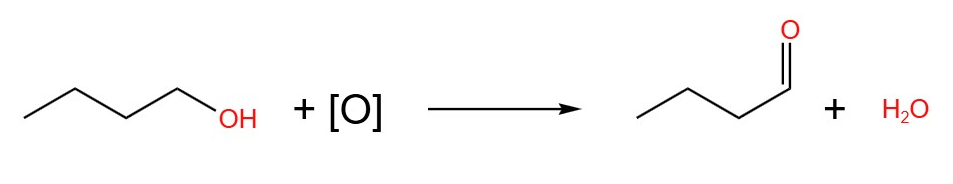
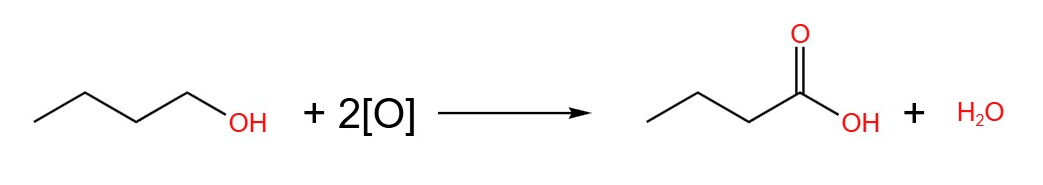
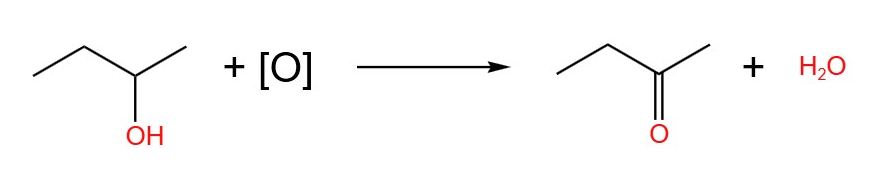
The equations of the oxidation of butan-1-ol, butan-2-ol and 2-methylpropan-2-ol are show below. The oxidising agent in these equations are given the placeholder [O].
- Butan-1-ol, immediate distillation:
- Butan-1-ol, reflux:
- Butan-2-ol, immediate distillation or reflux:
- 2-methylpropan-2-ol, immediate distillation or reflux:
DEHYDRATION:
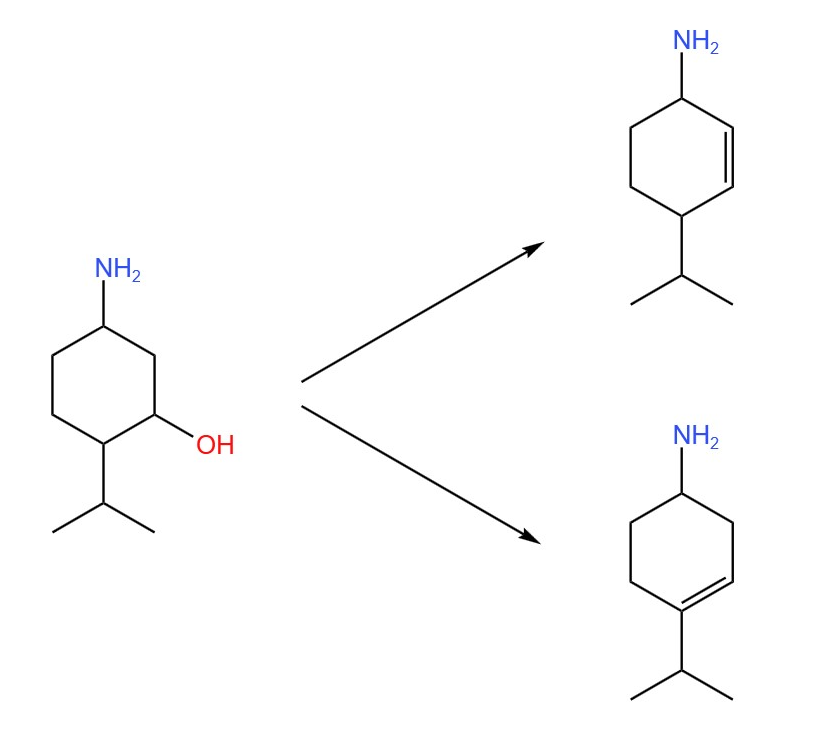
Just as alkenes can be hydrated to form an alcohol, alcohols can be dehydrated to form an alkene. This involves removing the OH group from its carbon and a second hydrogen from an adjacent carbon.
This results in a double bond being formed between the two affected carbons. If the carbon with the OH group is adjacent to multiple other carbons, then the second hydrogen can be taken from any one of them, randomly. This means that multiple products may be formed:
For this reaction, an acid catalyst and heat is used (the mechanism is not required for this course).
SUBSTITUTION:
One final reaction of alcohols is the conversion between them and halogenoalkanes (see 4.2.2 - Haloalkanes). Because the hydroxyl group in an alcohol is more stable than the halogen in a haloalkane, the reaction is unlikely to occur without the help of a sulfuric acid catalyst to be a source of H+ ions. The reagent for this reaction is a sodium halide, e.g. NaBr or NaCl.