4.1.3 - Alkenes
Properties of Alkenes
Alkenes are part of another relatively simple homologous series. They are still hydrocarbons, but the difference between them and alkanes is that alkenes are unsaturated. This means that there exists in the molecule at least one carbon-to-carbon (CC) double bond.
As explained in 2.2.2 - Bonding and Structure, the two bonds in a double are not the same. The first bond, a -bond, is the overlapping of s-orbitals directly in-between the bonding atoms and is the type of covalent bond seen in every molecule. The second bond, a -bond, is the sideways overlap of adjacent p-orbitals above and below the plane of the -bond. Because of the second anchoring force in a double bond, they have restricted rotation. Imagine two pencils together - trying to twist them around each other will cause one to break. This is the same with bonds. For a double bond to rotate, the -bond has to break and reform.
A carbon atom that is making a double bond with another carbon atom and two single bonds with hydrogen atoms is surrounded by three areas of electron density caused by four bonding pairs. Since the electrons in a double bond have the same repulsion as those is a single bond, the electron-pair repulsion theory suggests that the shape with least repulsion is trigonal planar, with bond angle 120.
Stereoisomerism in Alkenes
Stereoisomers are defined as:
Compounds with the same structural formulae but with different arrangements of atoms in space.
One type of stereoisomerism is geometric stereoisomerism, where there is really no difference in the properties of the two stereoisomers other than its geometry. This type of stereoisomerism arises from the restricted rotation of a carbon-to-carbon double bond.
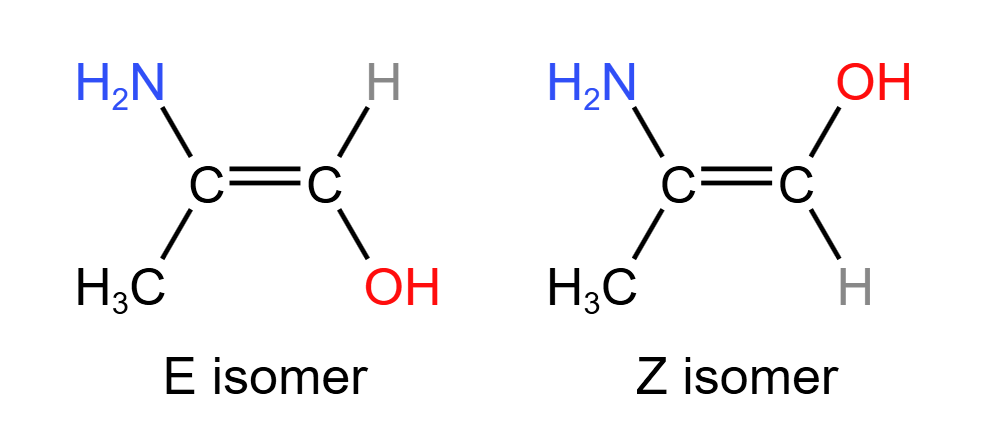
Geometric stereoisomerism may also be referred to as E/Z stereoisomerism or cis-trans isomerism. This is because the two variants are assigned either E or Z depending on which orientation their double bond has.
The second criterion for E/Z isomerism is that both carbons atoms involved in the double bond must be bonded to two different atoms or groups. This is because if one or both carbon atoms were bonded to two of the same atom, then there is no difference between the two possible orientations of the double bond
This can be simplified into:
E/Z isomerism - an example of stereoisomerism, in terms of restricted rotation about a double bond and the requirement for two different groups to be attached to each carbon atom of the CC group.
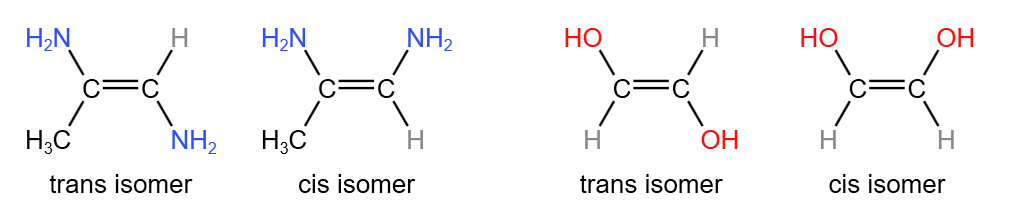
Cis-trans isomerism is a special case of E/Z isomerism. It involves the comparison between the groups/atoms attached to each of the carbon atoms in the double bond. Of the two groups on each carbon atom, if one or both from a carbon are the same as one or both from the other carbon, this is cis-trans isomerism. For example:
Since cis-trans isomerism is only a special case of E/Z isomerism, a molecule must show E/Z isomerism to show cis-trans isomerism. This means that a cis isomer is also a Z isomer, and a trans isomer is also an E isomer.
CAHN-INGOLD-PRELOG (CIP) PRIORITY RULES:
Just as with general nomenclature, the identification of different stereoisomers is standardised to ensure correct communication between all chemists. For this, chemists use rules called CIP rules, which determine whether a stereoisomer is E (entgegen - ‘in contrast to; contrasting’) or Z (zusammen - ‘together’), and refer to the atoms bonded to the CC functional group:
- For single atoms, a higher atomic mass gives a molecule higher priority. For example, bromine has a higher atomic mass than hydrogen and so has higher priority.
- For groups of atoms, look at the atom directly bonded to the carbons in the double bond. Whichever carbon is bonded to the atom with the highest atomic mass will have priority. If these are the same (for example if both are carbons), look down the chain.
- Stereoisomers with higher priority groups on the same side of the CC plane is a Z isomer. With higher priority groups on contrasting sides of the CC plane, a molecule is the E isomer.
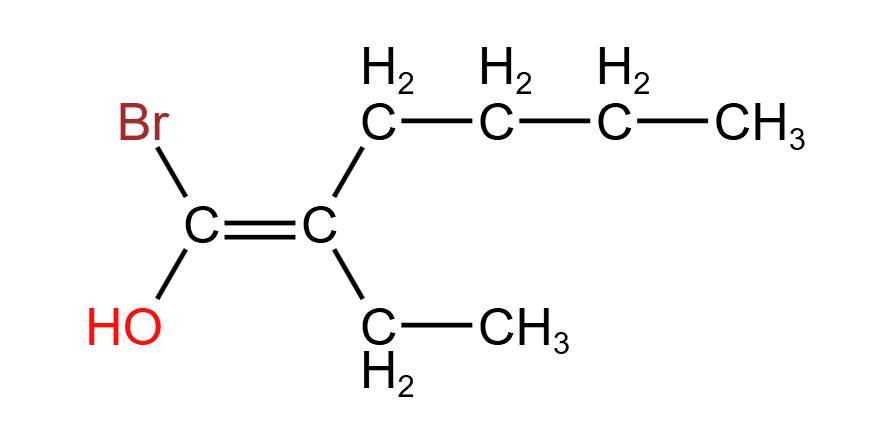
- Looking on the left, a comparison between bromine (79.9 amu) and oxygen (16.0 amu) yields that bromine is higher priority.
- Looking on the right, the two atoms bonded to the CC are both carbon. Looking further along the chains, we encounter another two carbons. Even further down, we encounter a hydrogen on the lower branch and a carbon on the upper branch. Since carbon (12.0 amu) is heavier than hydrogen (1.0 amu), the butyl chain is higher priority.
- This means that the two higher priority groups are both on the same side of the CC plane, making this molecule a Z isomer.
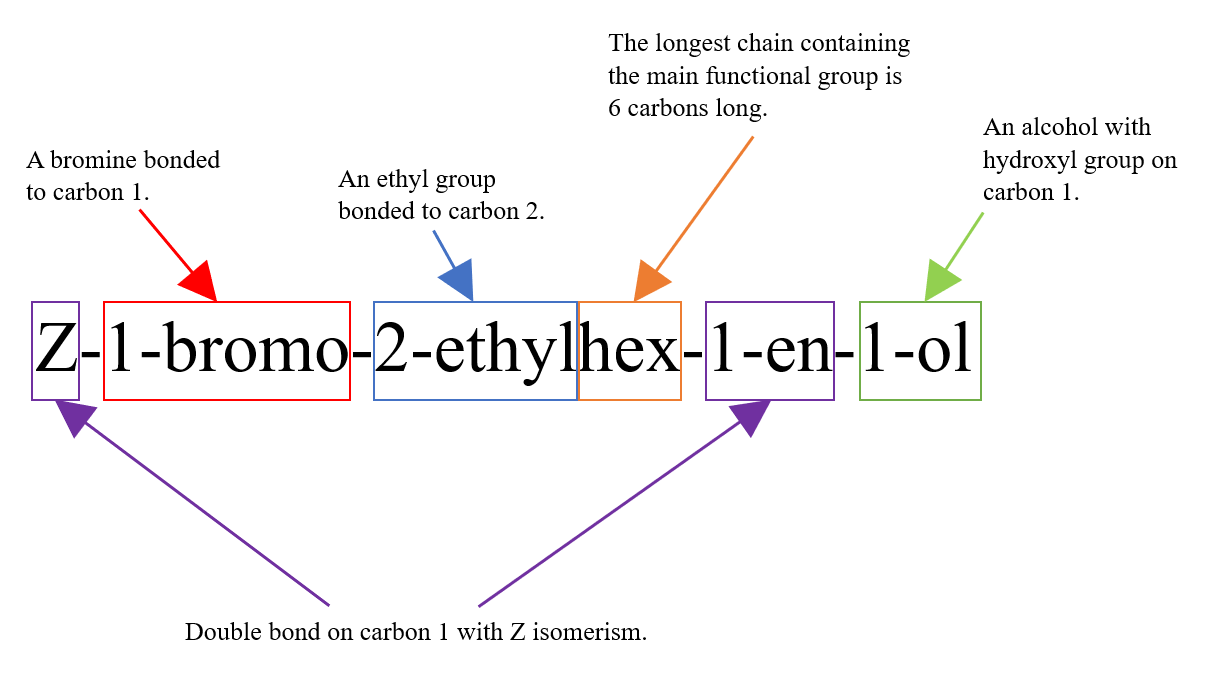
The full name of this molecule is Z-1-bromo-2-ethylhex-1-en-1-ol. Below is an aid that explains the meaning of this name:
Addition Reactions of Alkenes
Alkenes are more reactive than alkanes because of their double bonds. The -bond in a double bond has lower bond enthalpy than the -bond, meaning it is more susceptible to attack in a reaction.
Alkenes can react in four main reactions:
- Hydrogenation - the addition of hydrogen with a nickel (Ni) catalyst to form a saturated hydrocarbon, an alkane.
- Dihalogenation - the addition of diatomic halogens to form a saturated dihaloalkane. This is the basis of using bromine water to test for unsaturation. The aqueous bromine that makes the water an orange-brown colour comes out of solution to react with the alkene, thus the water turns colourless.
- Monohalogenation - the addition of hydrogen halides to form a saturated monohaloalkane.
- Hydrolysis - the addition of water with a phosphoric acid (HPO) catalyst to form a saturated alcohol.
Because the space between the carbon atoms in a CC contains four electrons and not the usual two, the double bond has a high electron density. Therefore the type of reactants that react with alkenes are electron deficient, as they react with alkenes to ‘steal’ the extra electrons.
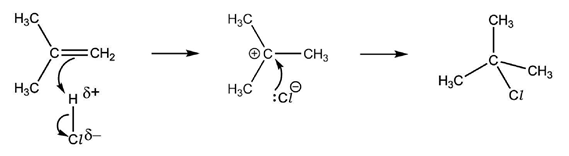
An electron pair acceptor is known as an electrophile. They have either partial or full positive charges, so the high electron density of the double bond attracts them and then the reaction occurs. The reaction mechanism by which this occurs is called electrophilic addition and involves heterolytic fission of a permanent or induced dipole.
When a diatomic molecule is close to the double bond, one of two things can happen. Either the molecule is polar to begin with, in which case it orientates itself so that the partially positive end is towards the electron-dense double bond: or it is non-polar, and coming close to the double bond will repel the electrons in the diatomic molecule’s bond, thus causing an uneven distribution of charges, polarising the electrophile.
Above is the reaction mechanism for the electrophilic addition of hydrogen chloride to 2-methylpropene, including curly arrows, all relevant dipoles and lone pairs.
MARKOWNIKOFF’S RULE:
The above mechanism is not the whole picture, because it shows the formation of only 2-chloro-2-methylpropane, whereas 1-chloro-2-methylpropane can also be formed. The reason why it is not shown is because the mixture of products at the end will have more of the 2-chloro-2-methylpropane than 1-chloro-2-methylpropane.
This is due to the stability of the intermediate compound. The reaction mechanism shows that a positively charged carbon compound, a carbocation, exists for just a little period while the rest of the mechanism has yet to happen. The chloride ion then attaches to the carbon with the positive charge via a dative covalent bond. This means that the position of the chlorine in the final product depends on which carbon the hydrogen from the electrophile did not bond to.
Alkyl chains have an electronic induction (electron-pushing) effect, so a positively charged carbon bonded to an alkyl chain will be more stable, because the positive charge is reduced by this electron-pushing. A positively charged carbon binding more than one alkyl chain will be even more stable.
Primary carbocations are carbocations in which the carbon with the positive charge binds one other carbon. They are the least stable type of carbocation.
Secondary carbocations are carbocations in which the carbon with the positive charge binds two other carbons.
Tertiary carbocations are carbocations in which the carbon with the positive charge binds three other carbons. They are the most stable type of carbocation.
In chemistry, stability is rewarded, and so the first step of an electrophilic addition will produce more of the most stable possible intermediate carbocation. As a result, more of one product will be formed than the other. The product that is more predominant is called the major product, while the other product is the minor product.
Polymers from Alkenes
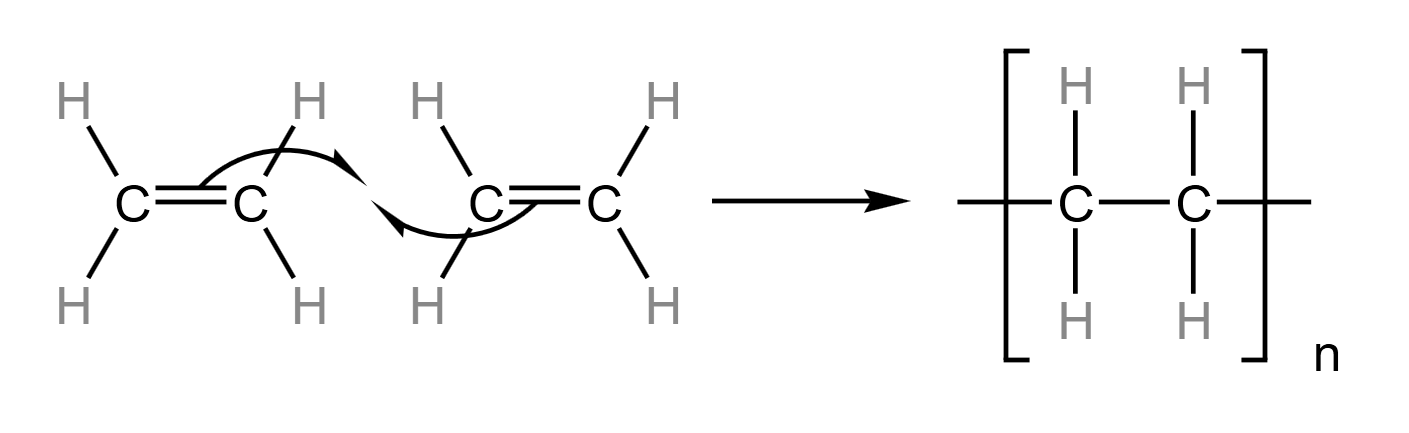
Another property of alkenes is that they can form polyalkenes. These are when the -bond breaks to form -bonds with adjacent alkenes. They can involve thousands of alkene molecules, and have upwards of ten thousand carbon atoms, so although they may look like alkanes - and have the same properties as alkanes - they are referred to as polyalkenes.
Polyethene is an example of a polyalkene, where the monomer (single molecule that is added to other identical molecules to make a polymer) is ethene. Shown below is the formation of polyalkene at a fine level:
This interaction occurs between every adjacent pair of ethene molecules, where ‘n’ is the number of repeat units in the polymer. For example, if you are asked to draw a polymer showing two repeat units, then make sure to write in the bottom right instead of n.
Because the two alkene molecules are added together, with no by-products, this type of polymerisation is called addition polymerisation.
The issue with this type of polymer is that there isn’t anywhere for enzymes in decomposers or rays from the sun to attack and break it down. As a result, they are neither biodegradable nor photodegradable. This causes many issues in nature, as disposal of addition polymers is effectively impossible. They can build up in landfill sites, and can end up in natural ecosystems and their food webs.
As a result, a massive push has occurred towards using plastics that are disposable and reusable, such as condensation polymers.