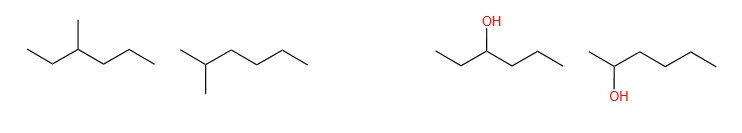4.1.1 - Basic Concepts in Organic Chemistry
Nomenclature
As with most of experimental chemistry, the naming of compounds is a standardised procedure. Nomenclature is a series of steps set out by the International Union Pure and Applied Chemistry (IUPAC) that ensure the consistent and unique naming of compounds. This section applies only to organic molecules - molecules (covalent bonding) whose main structure is based from a carbon spine.
The rules set out by the IUPAC are as follows:
- Identify the functional groups, distinguish the main one and assign a suffix (explained later).
- Identify the longest carbon chain containing the main functional group, and assign a stem shown in the table below.
- Number the carbons in this chain, starting at one end with 1, such that the main functional group has the lowest possible number.
- Identify any side chains and/or secondary functional groups on the longest carbon chain, and assign further prefixes that include which number carbon it is from.
- If multiple side chains/functional groups of the same type exist, the words in the prefixes include further prefixes pertaining to the number of them (di-, tri-, tetra- …)
- Numbers are separated by commas; numbers and words are separated by dashes.
| Length of Carbon Chain | IUPAC stem |
|---|---|
| 1 | meth- |
| 2 | eth- |
| 3 | prop- |
| 4 | but- |
| 5 | pent- |
| 6 | hex- |
| 7 | hept- |
| 8 | oct- |
| 9 | non- |
| 10 | dec- |
As for the suffixes for each functional group, the table below shows the order of precedence (importance) for the functional groups and their suffixes and prefixes. The prefixes are used when a more important group determines the suffix. To distinguish between saturated and unsaturated compounds, the ‘an’ shown below is replaced by ‘en’ when unsaturated:
| Functional group | Formula | Homologous series | Suffix | Prefix |
|---|---|---|---|---|
| Carboxyl | -COOH | Carboxylic acid | -anoic acid | - |
| Oxydiacyl | -COOCO- | Acid anhydride | -anoic acid anhydride | - |
| Ester bond | -COO- | Esters | -anoate | - |
| Acyl | -COX | Acyl halides | -anoyl halide | halocarbonyl- |
| Amide bond | -CON- | Amides | N-…-anamide | N-amido- |
| Cyanide | -CN | Nitriles | -ane nitrile | cyano- |
| Carbonyl | -CHO | Aldehydes | -anal | formyl- |
| Carbonyl | -CO- | Ketones | -anone | oxo- |
| Hydroxyl | -OH | Alcohols | -anol | hydroxy- |
| Amino | -NH | Amines | -anyl amine | amino- |
| Double bond | -C=C- | Alkenes | -ene | - |
| Alkyl | -CH- | Branched compounds | - | alkyl- |
| Halogen | -X | Halogen compounds | - | halo- |
EXAMPLE:
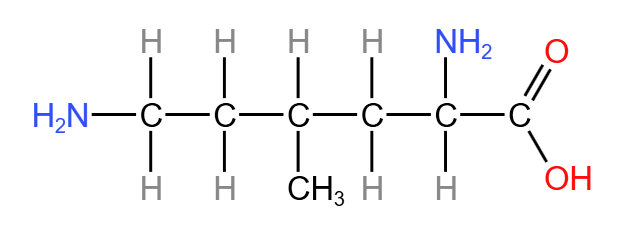
With these rules, the naming of the following compound can be seen below:
- The functional groups present are the two NH (amine) groups and the COOH group. Of the two, the COOH group is more important, with suffix ‘-anoic acid’.
- The longest carbon chain bonding to the COOH is 6 carbons long (including the one in the functional group).
- Numbering the carbons, the COOH is on carbon 1, the amine groups are on carbons 2 and 6, and the methyl group (extra carbon not in the main chain) is on carbon 4.
- This makes the prefixes ‘2,6-diamino’ and ‘4-methyl’.
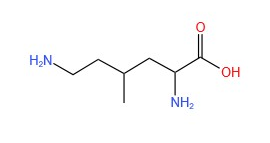
- The full name of the compound is therefore 2,6-diamino-4-methylhexanoic acid.
Formulae
Along with the standardised name of a compound, the atoms within can be expressed visually in varying degrees of accuracy. This means that one compounds has many types of chemical formulae that display the compounds:
- General formula: e.g. CH for alkanes. It is the simplest algebraic formula of a member of a homologous series.
- Structural formula: e.g. CHCHCHCH for butane. It is the minimal detail that shows the arrangement of atoms in a molecule.
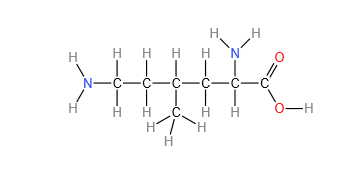
- Displayed formula: e.g. (see below for 2,6-diamino-4-methylhexanoic acid). It is the relative positioning of atoms and the bonds between them.
- Skeletal formula: e.g (see below for 2,6-diamino-4-methylhexanoic acid). It is the simplified organic formula, shown by removing hydrogen atoms from alkyl chains, leaving just a carbon skeleton and associated functional groups.
Functional Groups
For this topic, the definitions of several terms are important. In an exam, you will likely be asked the definition for homologous series, and the answer must be identical to the ones given below:
| Term | Definition |
|---|---|
| Homologous series | a series of organic compounds having the same functional group but with each successive member differing by CH |
| Functional group | an atom or group of atoms responsible for the characteristic reactions of a compound |
| Alkyl group | A branched carbon chain of general formula CH |
| Aliphatic | a compound containing carbon and hydrogen joined together in straight chains, branched chains or non-aromatic rings |
| Alicyclic | an aliphatic compound arranged in non-aromatic rings with or without side chains |
| Aromatic | a compound containing at least one benzene ring |
| Saturated | All carbon-to-carbon bonds are single bonds |
| Unsaturated | The presence of multiple carbon-to-carbon bonds, including CC, CC and aromatic rings |
Isomerism
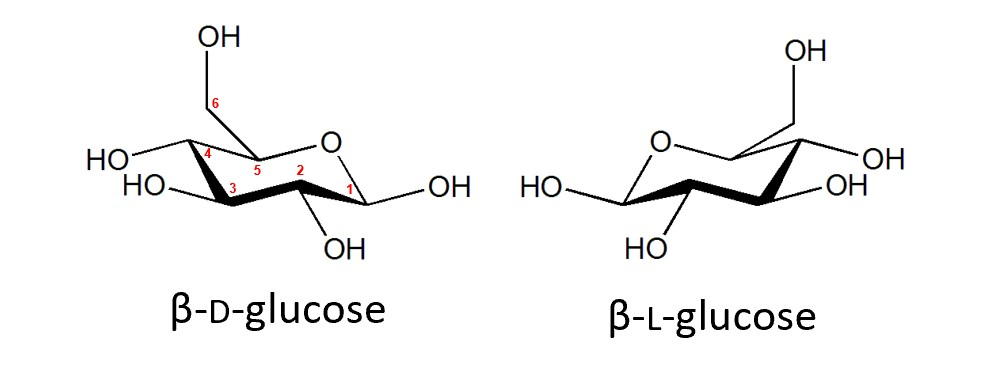
Isomerism is an important quality of some organic compounds, since the use of them in nature is highly selective. For example, an L-glucose molecule is the mirror reflection of a D-glucose molecule (this is optical isomerism, a topic in module 6).
However, D-glucose occurs more abundantly in nature, because the enzymes in photosynthesis do not produce L-glucose. As a result, enzymes in respiration only use D-glucose to release energy. The body cannot extract energy from L-glucose, and so it is useless. Another interest case about the differences in optical isomers is the Thalidomide Tragedy, where one isomer relieved morning sickness, and the other caused terrible birth defects in the soon-to-be children.
For now, the term structural isomer needs to be known:
Compounds with the same molecular formula but different structural formulae.
This differ from optical isomers in that optical isomers have the same structural formulae, but a different arrangement of atoms in space. For this reason, they are part of a larger group called stereoisomers (stereo- meaning ‘relating to a 3D effect’).
There are three types of structural isomers, which are:
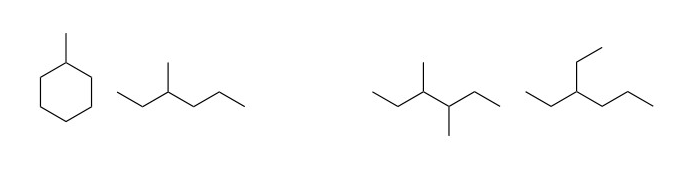
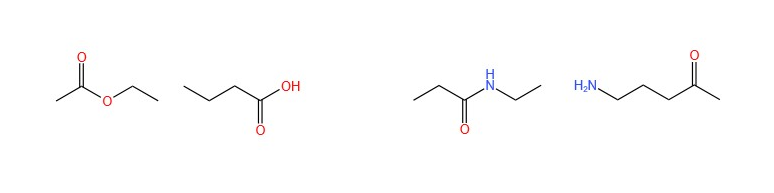
- Chain isomers, which differ in the level of branching and number of cycles:
- Positional isomers, which differ in the position of the functional group or branches:
- Functional isomers, which differ in the functional group of the compound:
Reaction Mechanisms
The progress of reactions is not instantaneous. In fact, a reaction may happen in many steps, and involve the forming/breaking of covalent bonds. Indeed, a single step in a reaction involving organic reactants may be the breaking of a known bond. As a result, a mechanism can be deduced and drawn to show how bonding pairs and lone pairs change hands in a reaction.
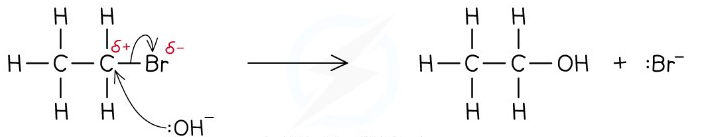
The drawing of a reaction mechanism may at first be difficult, because how do you show a moving electron or pair of electrons in a static image? The accepted way is using a curly arrow, where the number of heads on the arrow indicates the number of moving electrons. The tail-end indicates where the electrons come from, which is either a bond or a lone pair. The head of the arrow indicates where the electrons are going to, which is usually an atom with either a partial positive or fully positive charge.
Below is an example of a reaction mechanism where a bromine group is substituted for an -OH group.
FISSION:
When a covalent bond breaks, it can happen in one of two ways. Similar to formation, where one electron from each atom forms the same bond as if both electrons come from the same atom, a bond can break such that the electrons newly free from the bond is shared between the previously bonding atoms, or one of the atoms receive both of them.
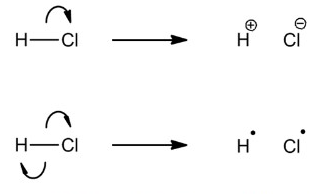
The breaking of a covalent bond is called fission and is either homolytic or heterolytic.
Homolytic fission is:
The breaking of a covalent bond such that each bonding atom receives one electron from the bonding pair, forming two radicals with equal charge.
The term ‘homolytic’ (homo- meaning ‘same’; -lytic meaning ‘breaking’) comes from the fact that the two bonding atoms become the same charge.
Heterolytic fission is:
The breaking of a covalent bond such that one bonding atom receives both electrons from the bonding pair, forming two ions with opposite charges.
The term ‘heterolytic’ (hetero- meaning ‘different’; -lytic meaning ‘breaking’) comes from the fact that the two bonding atoms become different charges.
Note - the tail of the curly should be originating at the bond. This diagram could be perceived as ambiguous.
In a covalent bond, the two atoms share a bonding pair because a paired electron is more stable than the otherwise unpaired electron. As a result, in homolytic fission, where the two bonding atoms receive only one of the pair, they become unstable and highly reactive. This highly reactive species is a radical - a species with an unpaired electron. A radical is denoted by a single dot.