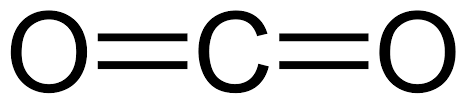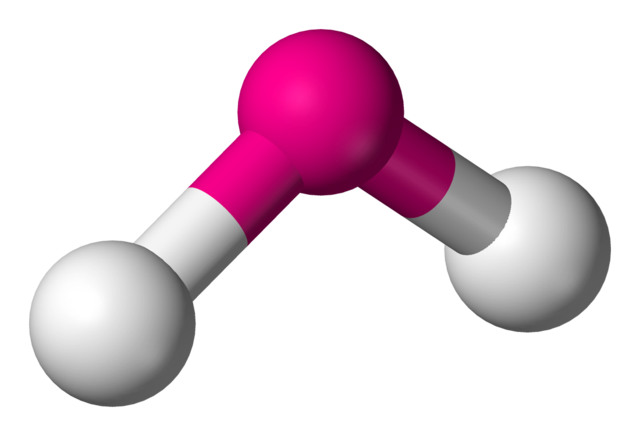2.2.2 - Bonding and Structure
Ionic Bonding
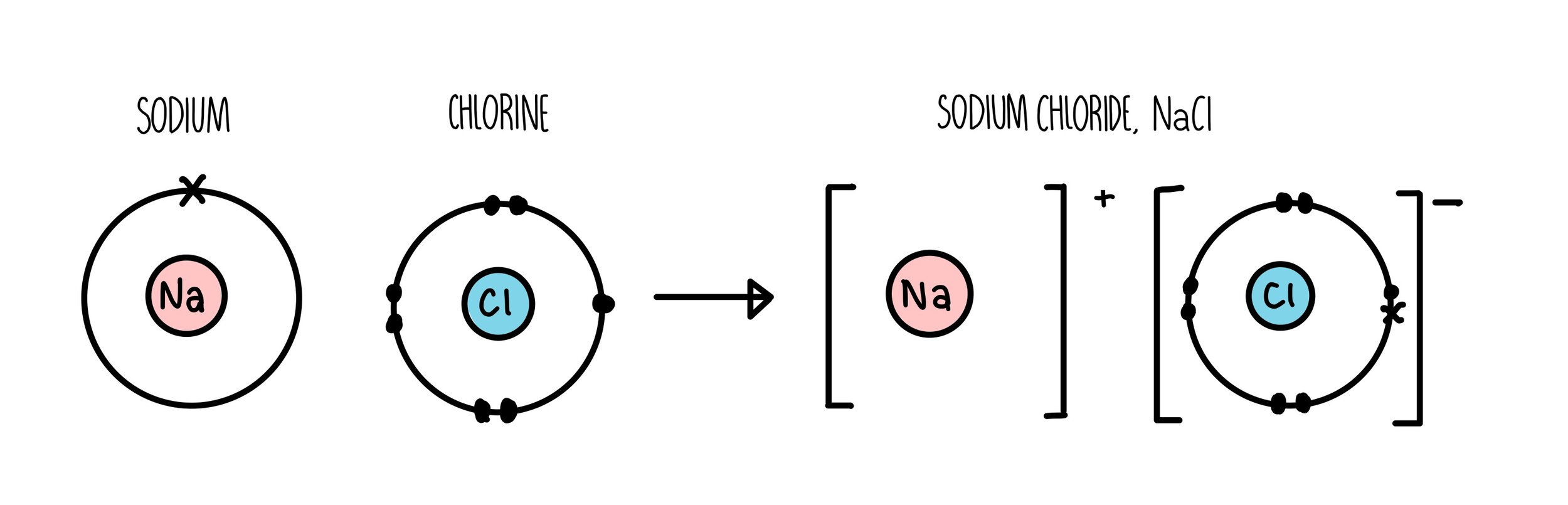
Ions have an overall charge caused by a disparity in the numbers of electrons and protons. Ions with opposite charges will have an electrostatic attraction between them. This is an ionic bond:
The electrostatic force of attraction between oppositely charged ions formed by the loss or gain of electrons.
Above is a dot and cross diagram which shows the atoms reacting to form one Na ion and one Cl ion. The extra electron in chlorine is the same one as the electron originally in the sodium, indicated by a cross amongst the dots, which represent chlorine’s electrons.
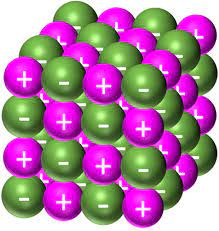
Ionic lattices are giant 3D structures that involve only ionic bonds, and alternate between cations and anions. Each ion has a strong electrostatic force from all sides, so ionic solids have high melting and boiling points. They are also relatively unreactive since the ions are stable.
They do, however, dissolve very readily in polar solvents like water, due to the opposite charges present. When dissolved or liquid, the ions are free to move. This means if a potential difference is introduced, a current will flow. In solid form, the ions are locked in a lattice and so are not free to conduct electricity.
Covalent Bonding
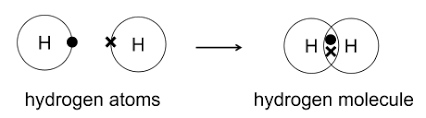
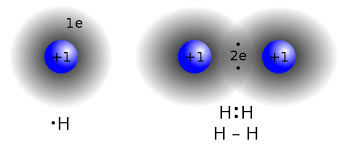
Electron energy levels are more stable when they are completely full than when they are partially full. To fill a level without causing an overall charge, atoms may share electrons, so the electrons instead orbit both nuclei, and thus overlapping their orbitals. This is a covalent bond:
The strong electrostatic force of attraction between a shared pair of electrons and the bonding nuclei.
Above is a dot and cross diagram of a covalent bond.
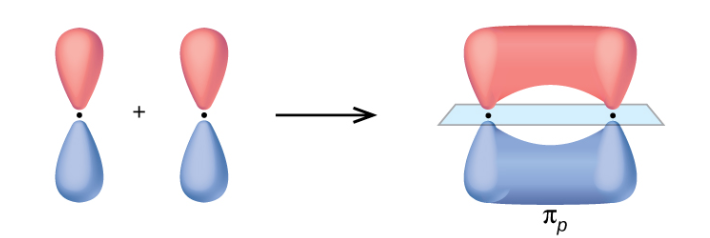
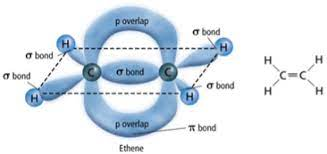
Above is a diagram displaying the merging of the two hydrogens’ s-orbitals. This introduces a new type of orbital. Since this is now one molecular orbital instead of two atomic orbitals, Greek letters are used. On the left is an s-orbital, on the right is a (sigma) -orbital.
If an additional pair of electrons is shared, the p-orbitals overlap sideways, producing a -orbital as well.
This produces a and a bond, a double bond.
It is also possible for an atom to contribute two electrons for a single covalent bond. This is common in transition metal chemistry and the reaction mechanisms of organic reactions.
A bond formed by this interaction is called a dative covalent bond, or coordinate bond, which is more accurately defined as:
A covalent bond in which both electrons involved come from the same atom.
The strength of a covalent bond is referred to as the average bond enthalpy:
The energy needed to break one mole of a given bond in a gaseous molecule.
and its units are .
For example, the average bond enthalpy of the oxygen to hydrogen bond (O—H) is 461 kJ mol. The higher the number, the stronger the bond, and vice versa.
Shapes of Molecules
Just like with magnets, like charges repel each other. This means that electrons will not get too close to each other, as they are both negatively charged, and so repel.
With this in mind, areas around an atom with a more dense arrangement of electrons, called regions of electronic density, will repel each other. These include:
- Single covalent bonds, as the region between the bonded atoms has a shared pair of electrons - a bonding pair - thus increasing the density of electrons. Two bonds from the same atom will form an angle, known as the bond angle.
- Multiple covalent bonds, (double, triple) as the region between the bonded atoms has multiple shared pairs of electrons in, so the electronic density is high. Single and multiple covalent bonds have equal electron repulsion. Bond angles also exist between these bonds.
- Lone pairs - a pair of electrons not involved in a bond, and still orbiting the nucleus - since they still have repulsive effects on other regions of electronic density. The repulsive effect of lone pairs are greater, as they are closer to the nucleus of the central atom, and by extension, to other electron-dense regions. Usually if a lone pair is instead of a bonding pair in molecule, it will reduce the bond angle between other bonds by 2.5.
By considering these three types of electron densities, each molecule will have a specific shape that places each region of electronic density as far apart as possible, thus reducing the repulsion between them.
Note: a molecule must have at least three atoms molecularly bonded to have a bond angle and a recognised shape.
For the following section, a bonding pair may refer to a single or multiple covalent bond.
LINEAR: Bond angle = 180
If a central atom has two regions of electronic density (two bonding pairs), then the shape with minimum repulsion and maximum distance is a straight line.
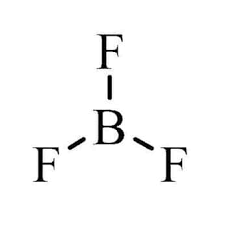
TRIGONAL PLANAR: Bond angle = 120
If a central atom has three regions of electronic density (three bonding pairs), then the shape with minimum repulsion and maximum distance is in a single plane, with the other atoms as the vertices of an equilateral triangle.
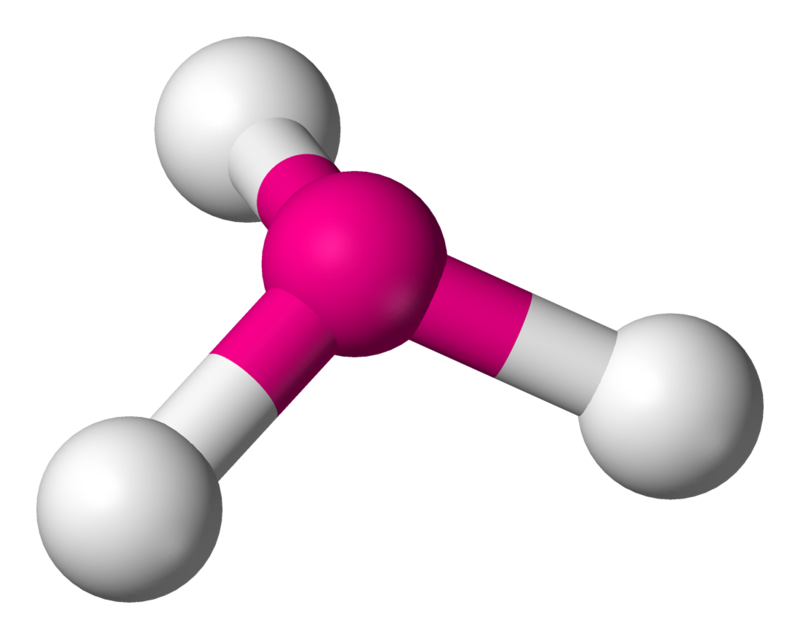
V-SHAPED/BENT: Bond angle = 104.5
If a central atom has four regions of electronic density (two bonding pairs, two lone pairs), then the shape with minimum repulsion and maximum distance is in a single plane, with the two bonds on one side of the atom, and the two lone pairs on the other.
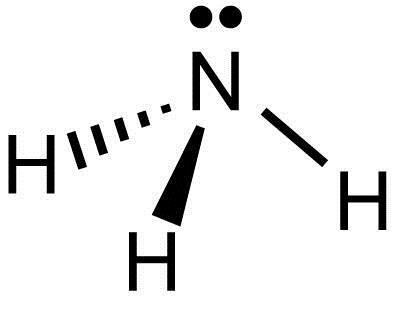
PYRAMIDAL: Bond angle = 107
If a central atom has four regions of electronic density (three bonding pairs, one lone pair), then the shape with minimum repulsion and maximum distance is not in a single plane, with the other atoms as the vertices of an equilateral triangle below the central one.
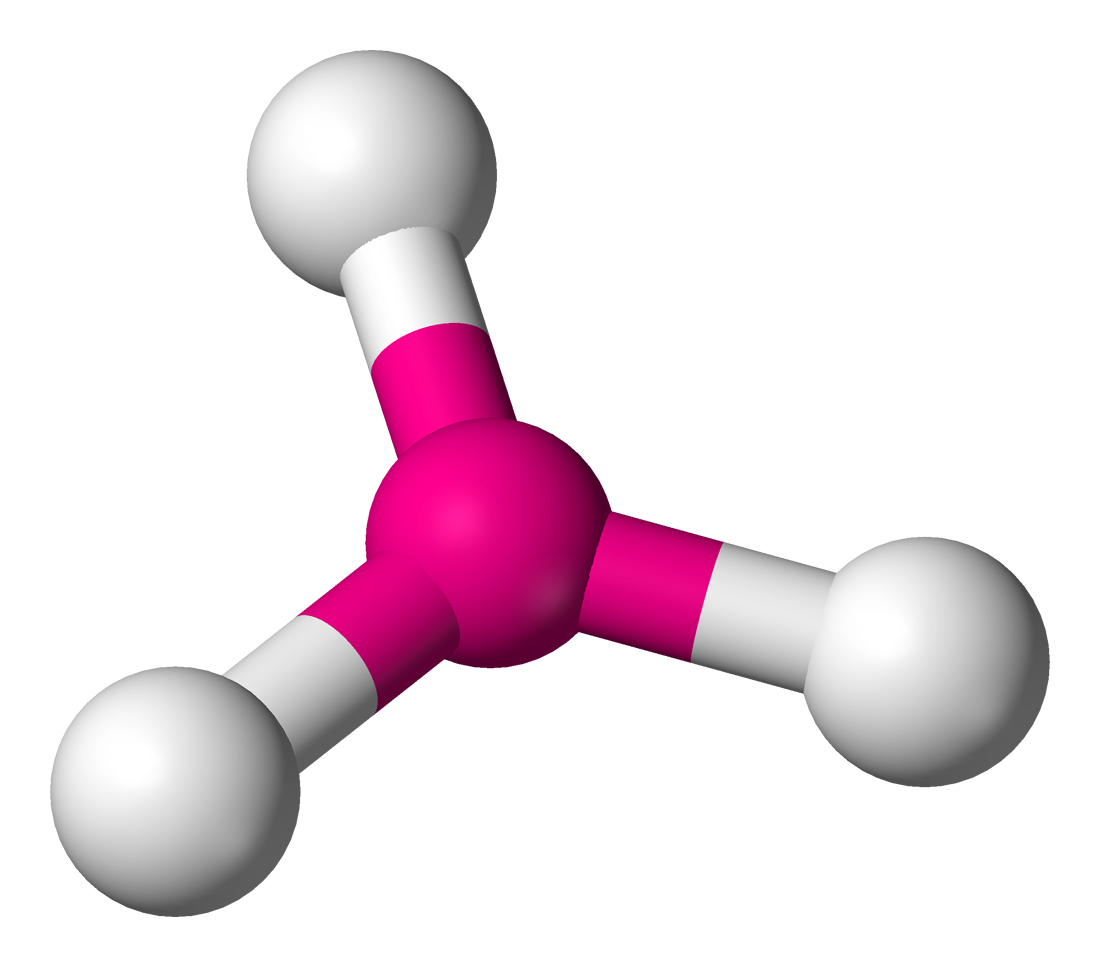
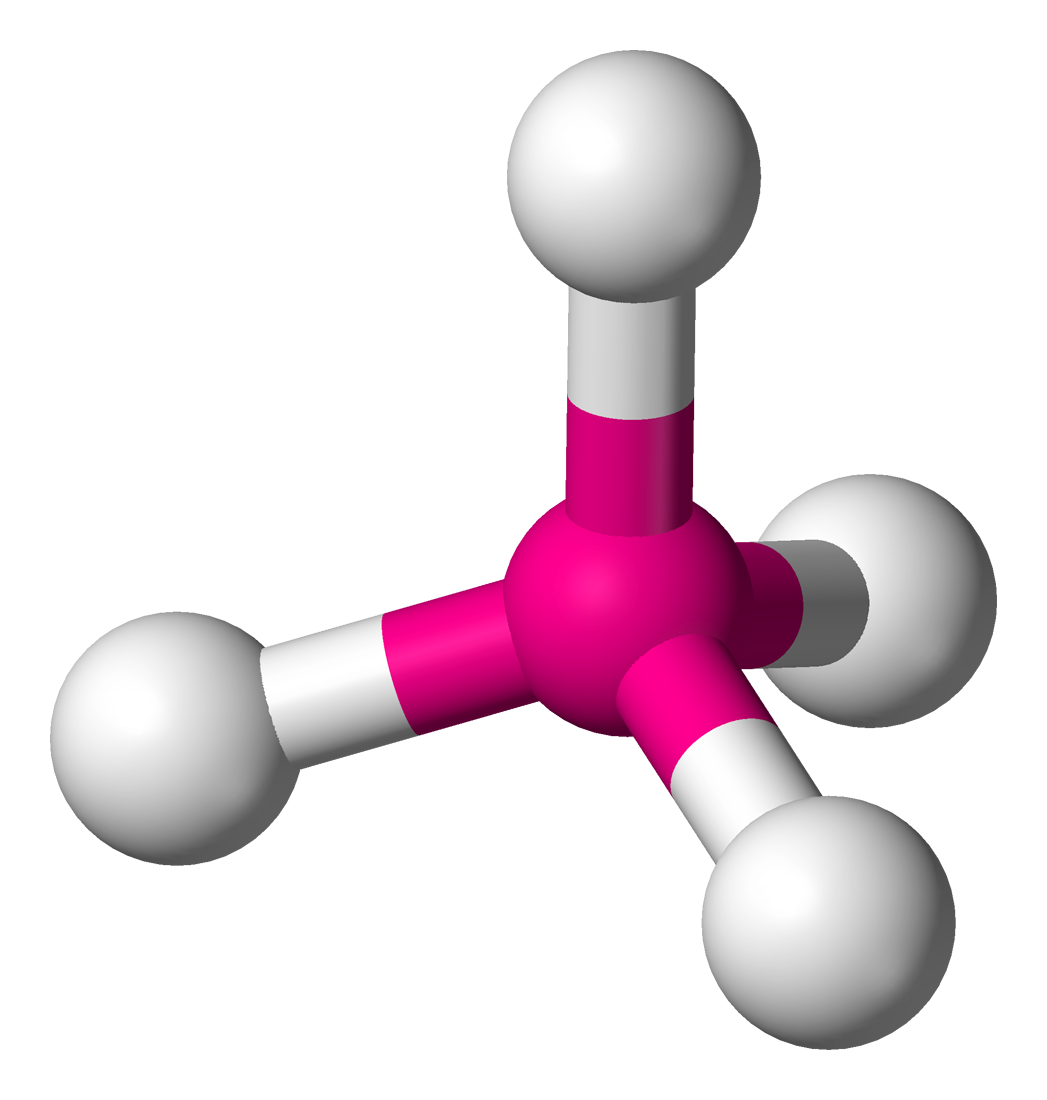
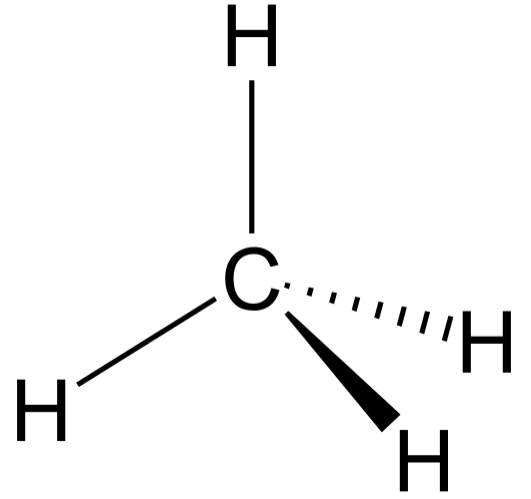
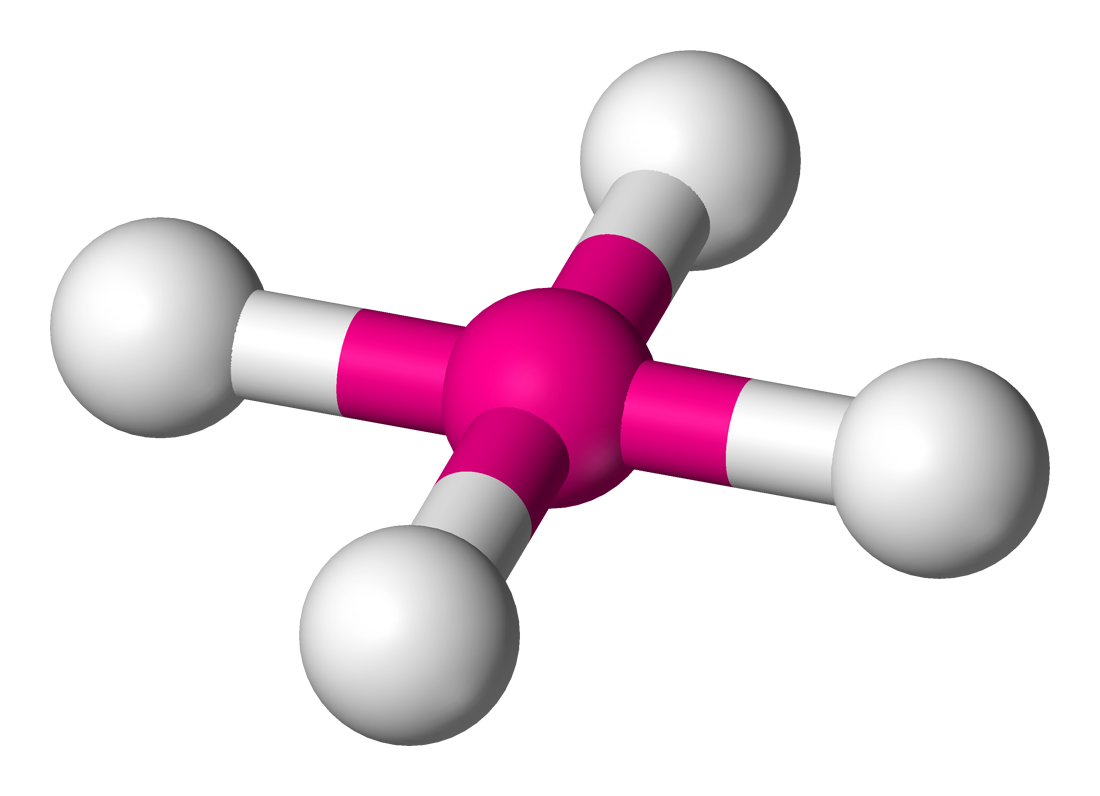
TETRAHEDRAL: Bond angle = 109.5
If a central atom has four regions of electronic density (four bonding pairs), then the shape with minimum repulsion and maximum distance is not in a single plane, with the other atoms as the vertices of a tetrahedron.
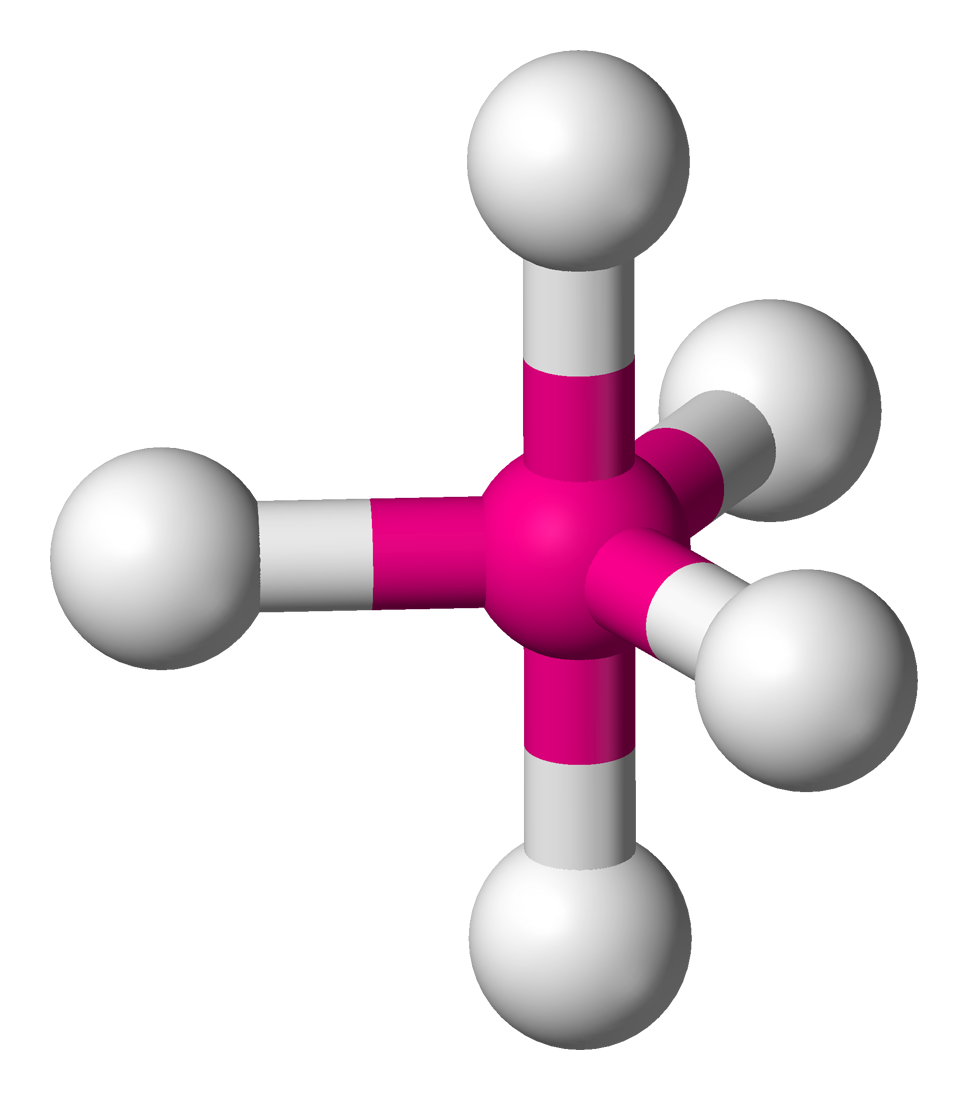
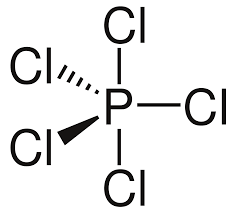
TRIGONAL BIPYRAMIDAL: Bond angles = 90 and 120
If a central atom as five regions of electronic density (five bonding pairs), then the shape with minimum repulsion and maximum distance is not in a single plane. There is a linear pole of three atoms and, perpendicular to the pole, a plane of atoms forming an equilateral triangle. The other atoms are the vertices of a triangular bipyramid (two tetrahedra together)
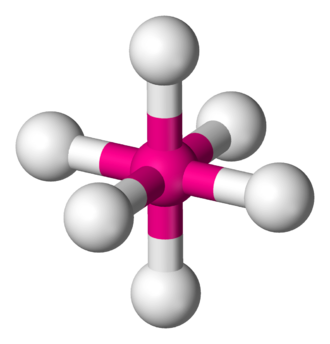
OCTAHEDRAL: Bond angle = 90
If a central atom as six regions of electronic density (six bonding pairs), then the shape with minimum repulsion and maximum distance is not in a single plane. There is a linear pole of three atoms and, perpendicular to the pole, a plane of atoms forming a square. The other atoms are the vertices of an octahedron.
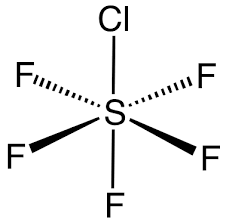
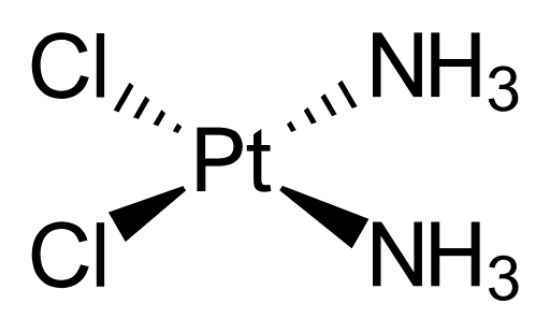
SQUARE PLANAR: Bond angle = 90
If a central atom as six regions of electronic density (four bonding pairs and two lone pairs), then the shape with minimum repulsion and maximum distance is in a single plane, lone pairs extending above and below, with the other atoms as the vertices of a square.
Cis-platin, a drug used in cancer treatment. It is a complex ion, encountered in transition metal chemistry. For now, ignore the fact that there is a covalent bond from a metal!
HOW TO DRAW A 3D STRUCTURE
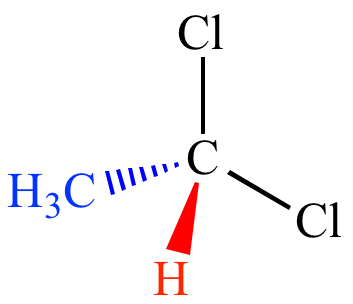
Obviously, when drawing a molecule on 2D paper, it’s difficult to construct a 3D image of it. Therefore there are conventions used to indicate whether a bond is in the same plane as the paper, or whether it extends above or below.
For a bond that is extending above the paper towards the observer, a solid wedge is used. For a bond extending below the paper away from the observer, a broken or dashed wedge is used. For a bond in the plane of the paper, a solid line is used
If a molecule is planar (trigonal planar, v-shaped and linear), all bonds are solid lines. Although square planar is planar, the convention is to draw two back-extending bonds and two forward-extended bonds.
Electronegativity
Since electrons are negatively charged particles, they can be influenced positively charged particles. This includes the electrons in covalent bonds, and the main thing influencing them are the nuclei of the bonding atoms.
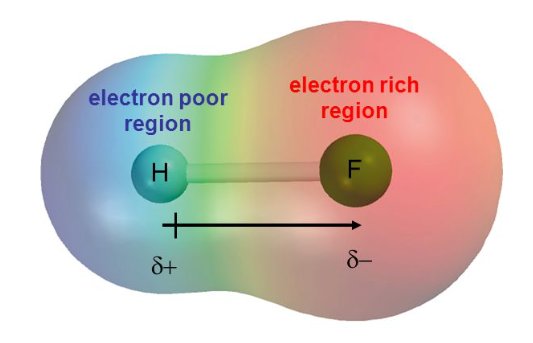
Let’s take a closer look at the bond between fluorine and hydrogen:
- Hydrogen and fluorine both donate one electron to form a covalent bond
- Hydrogen has one electron, but is more stable with two
- Fluorine has nine electrons, but is more stable with ten
- Hydrogen’s nucleus has one proton
- Fluorine’s nucleus has nine protons
This means that fluorine’s nucleus has a greater positive charge, and thus a greater inward pull on the electron pair involved in the bond than the hydrogen. This is known as electronegativity:
The ability of an atom to attract the bonding electrons in a covalent bond.
Electronegativity increases with group and decreases with period. Noble gases are not considered and so fluorine is the most electronegative atom.
Now, if fluorine is more electronegative, the electrons are going to be closer to the fluorine nucleus than to the hydrogen nucleus. But this means that hydrogen’s only electron is essentially pulled away, and all that’s left is a positively charged proton, making the hydrogen slightly more positive overall. Conversely, fluorine now has an extra electron orbiting closer, so is slightly more negative. The atoms aren’t fully positive or negative, so these are called partial charges, denoted with a Greek lowercase delta, and .
Since the centres of positive and negative charge are not superimposed (not in the same place), this bond is known as a polar bond, or a permanent dipole; and hydrogen fluoride the compound, is a polar molecule. A polar molecule is one in which all polar bonds do not cancel each other out. Carbon dioxide and water both have polar bonds, but in carbon dioxide they are symmetrical and the molecular centres of positive and negative charges are therefore superimposed.
Intermolecular Forces (Van der Waals’ Forces)
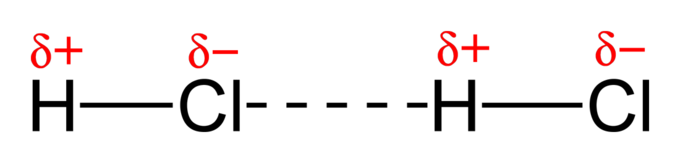
- Permanent dipole-permanent dipole interactions occur between two polar bonds. The partially negative end of one bond and the partially positive end of the other situate close to each other, causing an attractive force between the two oppositely partial-charged atoms. This is one of many interactions that hold two molecules together. Permanent dipole-permanent dipole interactions are present between chloromethane molecules.
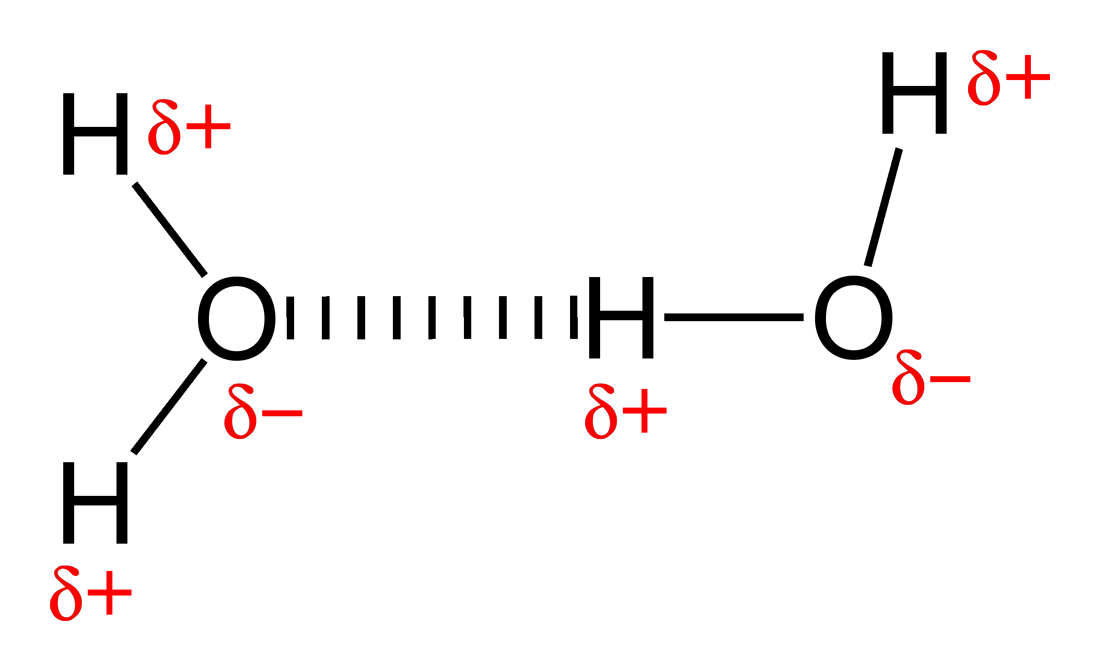
- Hydrogen bonds are special cases of permanent dipole-permanent dipole interactions. When hydrogen is bonded to a more electronegative atom, just a proton is left as one of the atoms in the bond. This can form a unique attraction to a lone pair of another atom, and is the strongest type of intermolecular force. Hydrogen bonds are present between water molecules.
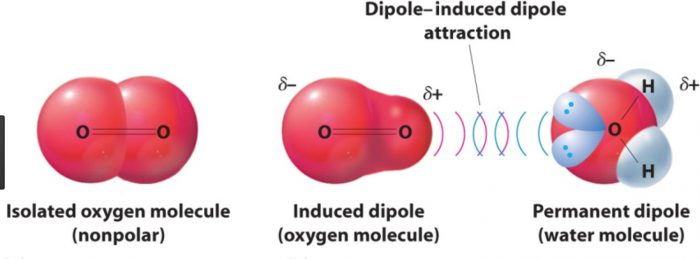
- Permanent dipole-induced dipole interactions occur when the partially negative end of a polar bond repels the electrons in a neutral (non-polar) bond. This is called an induced dipole, and induces a partially positive charge on one of the atoms. After this occurs, a permanent dipole-induced dipole interaction works identically to a permanent dipole-permanent dipole interaction. They are present between water and oxygen molecules.
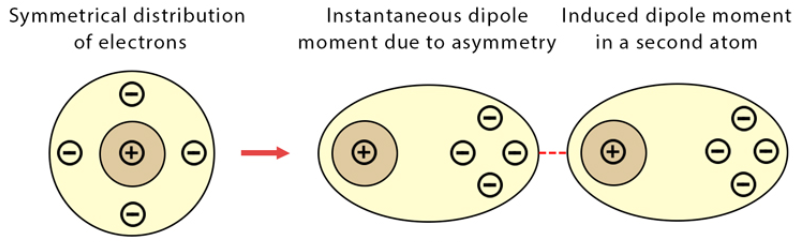
- Instantaneous dipole-induced dipole interactions occur when the random movement of electrons around an atom’s nucleus or in a covalent bond results in a very short-lived difference in the locations of the centres of positive and negative charges. This is called an instantaneous dipole. This temporary dipole induces a dipole in another atom or molecule, functioning very similarly to permanent dipole-induced dipole interactions. These occur between every molecule and atom, are the weakest type of intermolecular force, and are also known as London Dispersion Forces (LDFs).
Properties of Water
Due to the hydrogen bonding between water molecules, there are many properties of water than are perhaps counterintuitive.
For instance, normally a solid has densely packed particles, whereas in a liquid they are more spaced apart and randomly assorted. This is why a solid usually sinks in its liquid counterpart, since the solid is more dense. In water however, the hydrogen bonds in the simple covalent structure introduce a space between water molecules when it solidifies into ice. Therefore the water molecules are further apart than in water. This is why ice floats on its liquid counterpart.
Below is a video explaining the properties of water in a biological context. This area is also heavily emphasised in biology, as it is part of Biochemistry.